Antibody Diversity
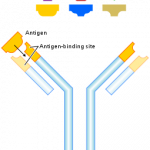 There are many different types of proteins that get made inside of human cells, including structure proteins, such as keratin and collagen, enzymes, and messenger proteins such as hormones. One large group that I forgot to mention, and that intrigues me the most, is the wide variety of different antibodies that get made in our blood cells. There are over a billion different types of antibodies that get made over the course of a lifetime, and each is able to identify antigens from foreign objects, such as viruses or bacteria, and elicits an immune response.
There are many different types of proteins that get made inside of human cells, including structure proteins, such as keratin and collagen, enzymes, and messenger proteins such as hormones. One large group that I forgot to mention, and that intrigues me the most, is the wide variety of different antibodies that get made in our blood cells. There are over a billion different types of antibodies that get made over the course of a lifetime, and each is able to identify antigens from foreign objects, such as viruses or bacteria, and elicits an immune response.
The amazing part of antibody production is the fact that the instructions on how to make so many of them are found in the DNA. DNA is divided up into segments, called genes, which have the instructions on how to make proteins. If there are only about 23,000 genes in human DNA, how do our cells make so many different types of antibodies? The number of antibodies exceeds the coding capacity of DNA tremendously.
This brings up a whole list of events that leads to antibiotic diversity, including the recombination of gene segments in the production of the protein. Multiple gene segments will recombine in the blood cells to form the heavy and light chain in the antibody. Just to give you an idea about how diverse they can be, the heavy chain itself has almost 11,000 different combinations that can result from the recombination of all of the gene segments. After a variety of antibodies are produced, random somatic mutations will occur which lead one specific antibody being able to bind to the antigen that is present.
To learn more about how cells read the instructions in DNA to make proteins, see http://www.dnai.org/a/index.html.
| Print article | This entry was posted by Jennifer Galasso on January 4, 2010 at 2:06 pm, and is filed under DNA Interactive. Follow any responses to this post through RSS 2.0. You can skip to the end and leave a response. Pinging is currently not allowed. |









