Catching the Flu: Between Complacency and Fear
It is “flu season” again and people are being vaccinated against the seasonal flu. But how do these vaccines work and why do we have to get a new one every year?
And how does this relate to the issue of scientific publishing and biosecurity? Read on…
Flu – or influenza – is a serious respiratory illness (not to be confused with common cold) caused by the influenza A and B viruses. Influenza claims a death toll of about 250,000 to 500,000 people worldwide every year. Medical advances have helped understand and combat one of history’s worst killers. From 1918 to 2012 the number of death from influenza has significantly decreased.
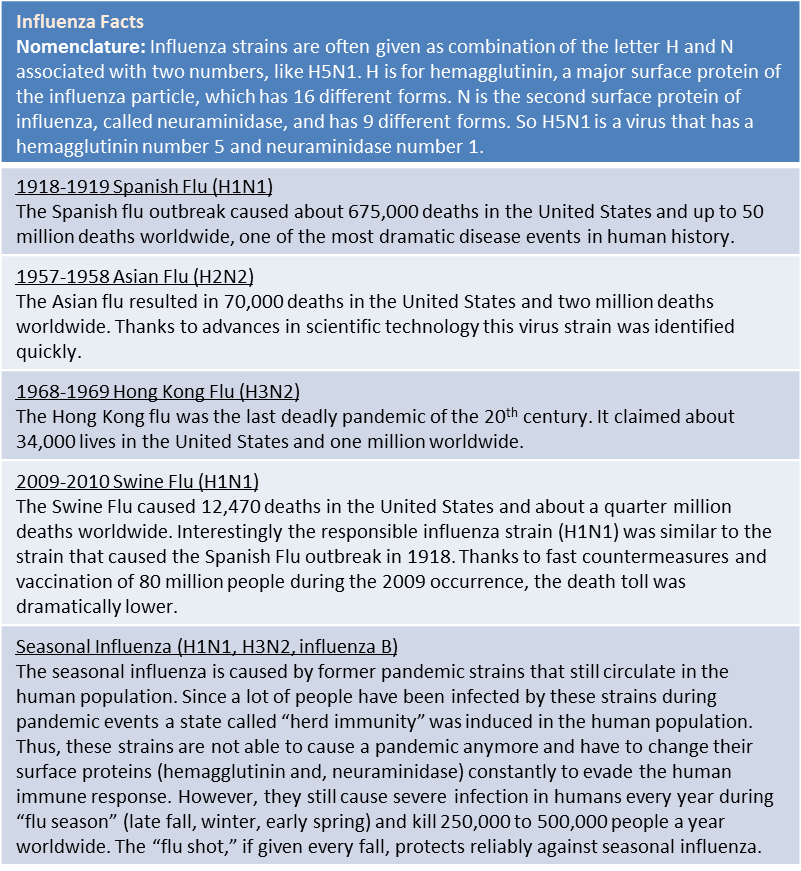
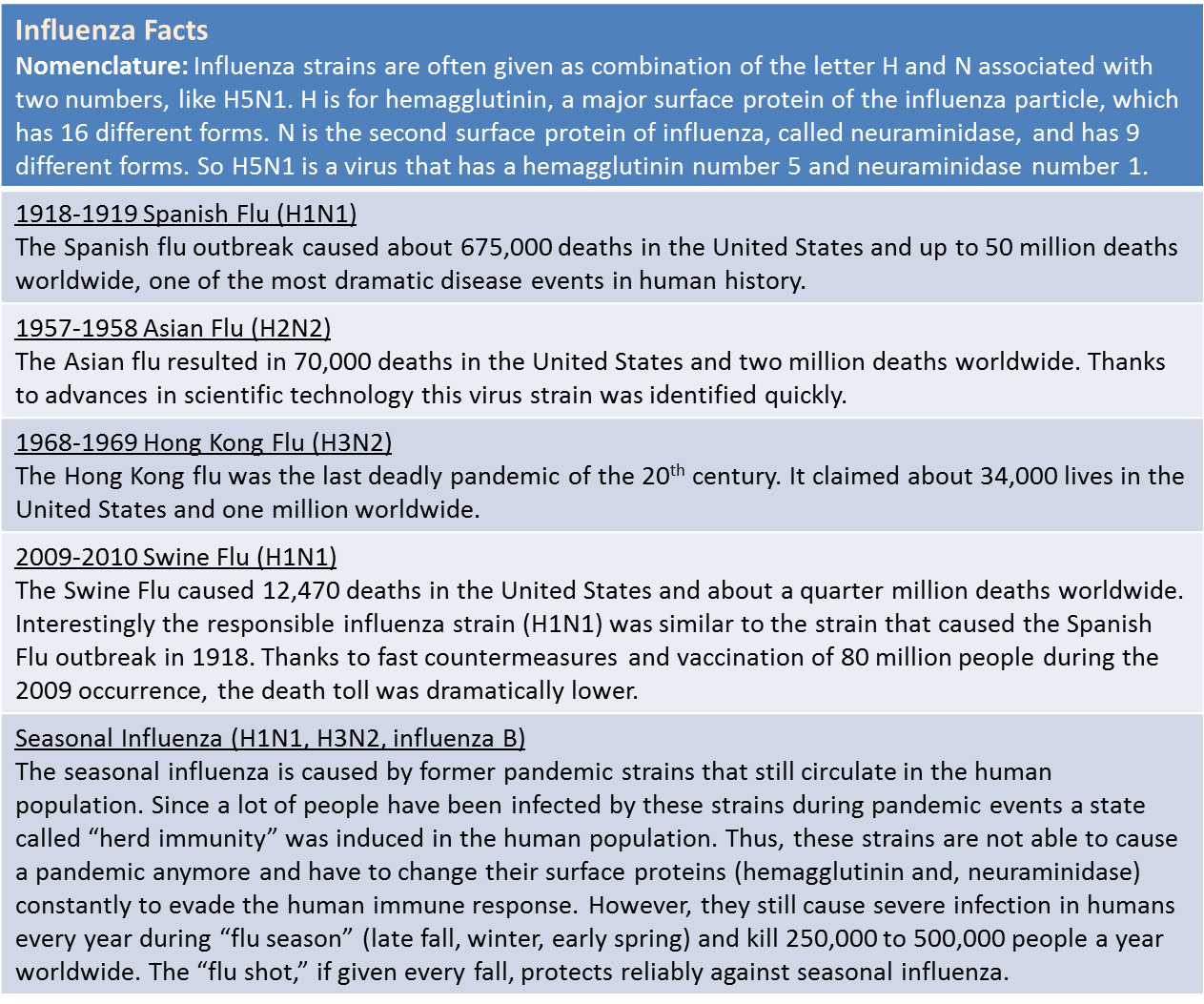
Box 1: Facts About Influenza. Source: www.flu.gov
Because the influenza virus can constantly mutate, it remains a highly challenging illness: there is no other vaccine that has to be changed on a yearly basis. Viruses evolve at a much faster rate than other organisms and scientists and pharmaceutical companies therefore have to keep updating their vaccines in response. And because evolution has produced variable strains of influenza, there is always a risk of predicting and targeting the wrong strain, which might result in an ineffective vaccine. Usually, the vaccine strains for the Northern hemisphere for the following season are picked in early spring based on data from the latest flu isolates from the Southern hemisphere and vice versa in fall. (This is the reason why vaccine strains for the Northern hemisphere are often called Brisbane or Perth – after the city in which they were isolated in Australia).
Additionally, animal influenza viruses jump from time to time into the human population, and can cause new pandemics. Candidates for such viruses are mostly avian viruses, most prominently the highly pathogenic H5N1 virus or “bird flu” (first isolated in Hong Kong in 1997) but also H9N2 and H7N7 viruses that infect humans sporadically. This scenario of avian viruses jumping into humans caused the pandemics of 1918, 1957 and 1968 (see Box 1) and can be imagined quite realistically in the recent movie, Contagion.
However, the avian H5N1 virus is unable to spread from human to human, an ability that is crucial for viruses to cause a pandemic. So how does a pandemic of avian flu occur?
Two research groups, Ron Fouchier’s group in Rotterdam in the Netherlands and Yoshihiro Kawaoka’s group in Madison, Wisconsin, addressed this question. They used ferrets as a model to look at what it takes to make the virus transmissible from human to human. They found that just a couple of mutations were necessary to enable the virus to spread from ferret to ferret. Ferrets are commonly used as model animals for influenza virus since they react to the infection in almost the same ways as humans. All of the mutations found in the mutant virus were also found in H5N1 viruses isolated from waterfowl and humans, but have never been found together on one virus.
So far, this is a pretty straightforward story of scientists figuring out how a virus works and developing a vaccine and efficient countermeasures to prevent it. But here’s the twist to this story, where researchers’ right to publish classed with government concerns about biosecurity.
Fouchier and Kawaoka simultaneously sent their manuscripts to the journals Nature and Science to share their findings with their international colleagues. However, strong concerns by the National Science Advisory Board for Biosecurity (NSABB) in the US about safety and bioterrorism forced both research groups to omit crucial information about the mutations from their manuscripts. The NSABB recommended giving access to the full information to a small group of approved scientists, but not the public.
This decision to forbid researchers to publish in full created debate and raised opposing viewpoints around the globe.
On the one hand, the US government takes the possible use of viruses in bioterrorism very seriously, especially as such a virus could easily be developed. The scientific community, on the other hand, considers the current situation as a crucial “catch-up” to help plan for future vaccines. In the January 2012 issue of Nature the restriction of access to the scientific methods and data is stated as “akin to censorship, and counter to science, progress and public health.”
In a huge effort researchers from all over the world monitor and sequence hundreds of influenza samples from all kinds of animals and humans on a daily basis. Since these dangerous mutations are now known, the scientific community can react quickly in case similar mutations are found in an isolated virus. The earlier such a virus is identified, the faster countermeasures can be taken, and the faster vaccines and antivirals can be produced. However, if the necessary information is restricted and not available to the scientific community this will slow down identification of highly transmissible forms of influenza and would delay countermeasures taken by health agencies.
Now, the scientific community around the globe has to find a way to cope with both, biosafety concerns on the one hand and scientific progress and benefits for public health on the other hand. Withholding important information from scientific colleagues may not be the solution but biosafety concerns have to be addressed as well. A scientific advisory board of leading influenze researchers could potentially judge how to deal with the release of crucial but delicate data.
This is an astonishing example of a new virus is changing scientific culture and human behavior without infecting a single individual. The current international debate about the free flow of scientific information and how to balance public health and biosecurity has the potential to change life science forever.
Links to background information and controversy comments over the H5N1 research result:
Most recent letters to Science by active participants of the discussion (January, 19th, 2012):
Ron Fouchier (Author of one of the H5N1 studies)
Daniel Perez (Influenza expert)
Michael T. Osterholm (NSABB board member)
Publications
Nature 480, 421–422 (22 December 2011) | doi:10.1038/480421a
Nature online 20 January 2012 | doi:10.1038/481443a
Science, Science Insider online 20 January 2012
Blog
Vincent Racaniello’s Virology blog and podcast:
| Print article | This entry was posted by Christine Marizzi on January 25, 2012 at 6:55 pm, and is filed under G2C Online. Follow any responses to this post through RSS 2.0. You can leave a response or trackback from your own site. |