Designer Biology in the Fight against Cancer
As many of you know and I have discussed before, cancer therapies have to target cancer cells while minimizing damage to normal cells. This is very difficult, in part because cancer cells are, although altered, still human cells. Synthetic biology is a new field that engineers biological systems for different applications. One interesting area where synthetic biology is now being applied is to cancer therapies. One advantage is that it may be possible to engineer sensors that can differentiate normal cells from tumor cells.
Early approaches have used engineering to create bacteria that specifically invade tumor cells. In one approach, bacteria were engineered to invade cancer cells. First, E. coli was given the ability to bind to and enter cancer cells by genetically engineering them to express a surface protein called invasin from another species of bacteria. Invasin binds specifically to beta1 integrin, which is a protein that is often expressed on the surface of cancer cells. After binding, the cells engulf the bacteria. However, other cells also express beta1 integrin, so E. coli that express invasion at all times can invade multiple cell types. To avoid targeting non-cancer cells, the expression of invasion was engineered so that it is only on when the bacteria are in areas with low oxygen, which was accomplished by hooking up the DNA coding for invasion to DNA that turns on gene expression only in apoxic e
nvironments. These low oxygen areas are often found within tumors, where the number of blood vessels is lower than in normal tissue. So, by combining two functions- one that allows invasion of a cell and one that targets tissue with the right characteristics, tumor cells can be selectively infected.
A new study takes advantage of recent advances in our understanding of the relationship of small RNAs, called microRNAs (miRNAs), and cancer cells. It turns out that different tumors express distinct levels of miRNAs, which are small RNAS that are important gene regulators. In fact, measuring the level of expression of different miRNAs can be used to diagnose certain cancers. This provides an opportunity: if a biological system could sense the levels of miRNAs and determ
ine if they matched a cancer profile, this could be used to target those cells.
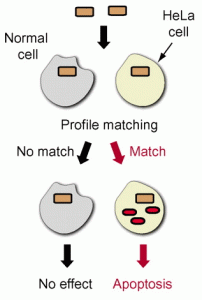
The biology of miRNAs makes this possible: miRNAs bind to target RNAs and affect gene expression. So, for any given miRNA, it is possible to engineer a target RNA that should be affected by it. To this end, Zhen Xie and colleagues developed a system that can sense whether the right combination of microRNAs is turned on or off in a cell and, if so, program the cell to commit suicide. In essence, Xie designed a biological logic circuit that senses HeLa cells, a cervical cancer cell line. HeLa cells have a microRNA signature where two microRNAs are over-abundant and three are under-abundant. Xie made his circuit so that it will only be turned on when the complete signature is present: if just one of the microRNAs that is over-abundant is missing or even one of the under-abundant microRNAs is present, the circuit doesn’t turn on- because the cell isn’t a HeLa cells. The circuit controls the expression of hBAX protein, which kills the cell. So, if and only if the cell has the right signature to be a HeLa cell, it will be killed.
The beauty of the system is that it could be adapted to work for many cancer cell types, and for other diseases, too. As long as a diagnostic profile of miRNAs can be identified, a logic circuit reading that profile should be possible.
Perhaps, in the future, these two approaches can be combined, using engineered bacteria to introduce a biological computer into certain cells, and then using that computer to monitor whether those cells have dangerous characteristics which need to be altered.
For more information, see Xie, et al. Multi-Input RNAi-Based Logic Circuit for Identification of Specific Cancer Cells. Science 333:1307-1311 and Anderson, et al. Environmentally Controlled Invasion of Cancer Cells by Engineered Bacteria. Journal of Molecular Biology 355:619-627.
| Print article | This entry was posted by Bruce Nash on November 8, 2011 at 5:28 pm, and is filed under Inside Cancer. Follow any responses to this post through RSS 2.0. You can leave a response or trackback from your own site. |